Abstract
Background: Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) and the most common indolent NHL. Rituximab-based therapy has been a mainstay in FL treatment, improving response rates, time to progression, and overall survival. Despite these improvements, most patients with FL will still relapse after frontline therapy, and therapies with curative potential are still elusive. Patients who relapse will experience poorer outcomes than those who do not and will continue to progress through successive lines of therapy (LOTs). The approval of treatments (eg, obinutuzumab and lenalidomide) may offer patients novel therapeutic options with the goal of improving outcomes in relapsed/refractory (R/R) FL. In this analysis, changes in treatment patterns and outcomes were assessed for the time periods from 2015 to 2017 compared with 2018 to 2021 to understand the impact of these new therapies on R/R FL.
Methods: This retrospective, observational study used US patient electronic medical records (EMRs) and claims data from Optum Market Clarity. Inclusion criteria were ≥ 1 diagnosis of FL between January 1, 2015, and December 31, 2021, from EMRs or ≥ 2 diagnoses of FL from claims (index date = first diagnosis); no FL or other primary cancer diagnosis 12 months prior to the index date (except unspecified NHL and benign prostate cancer); ≥ 12 months prior enrollment/clinical activity, without a diagnosis of diffuse large B-cell lymphoma after first diagnosis of FL; at least 1 LOT after the index date; and ≥ 6 months of follow-up. Treatment regimen utilization and overall survival (OS; defined as the time from initiation of an LOT to death) was analyzed using the Kaplan-Meier method, and patients were censored at the end of enrollment period/last clinical activity. Log-rank tests were used to evaluate survival differences stratified by time period for each LOT.
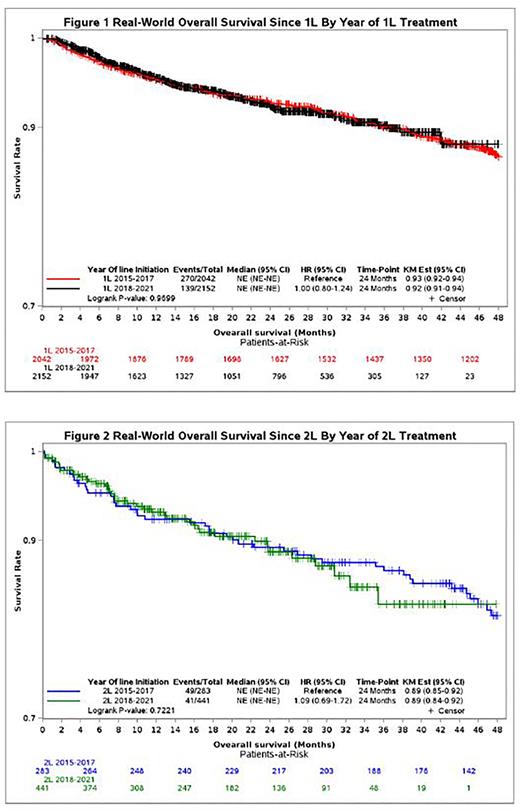
Results: A total of 2042 patients had 1 LOT, and 283 progressed to ≥ 2 LOTs between 2015 and 2017. For the 2018 to 2021 period, 2152 patients had 1 LOT and 441 progressed to ≥ 2 LOTs. The most prevalent first-line (1L) treatments across both time periods were rituximab-based therapies (2015-2017 vs 2018-2021: rituximab monotherapy [31.7% vs 30.0%], rituximab and bendamustine [41.1% vs 36.6%], and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [12.4% vs 12.2%]). Rituximab and bendamustine and rituximab monotherapy were also the most frequently used in second-line (2L) therapy and later during both time periods. Comparing the periods 2018 to 2021 with 2015 to 2017, increased utilization was observed for obinutuzumab-based therapies (1L: obinutuzumab and bendamustine [6.8% vs 0.8%]; ≥ 2L: obinutuzumab monotherapy [3.2% vs 0%]) and rituximab and lenalidomide (≥ 2L: 8.4% vs 3.9%). PI3K inhibitor utilization remained low across both time periods (1.1% [2018-2021] vs 0.7% [2015-2017]). Despite the introduction of new therapies, 2-year OS rates were similar across both time periods for patients receiving 1L and ≥ 2L therapy (Figures 1 and 2).
Limitations: Optum Market Clarity does not have physician-assessed progression events; thus, LOT advancement in this analysis was based on treatment-regimen change and may not necessarily indicate disease progression. With all real-world studies, there are inherent limitations due to unmeasured confounders and censoring because of loss to follow-up; however, these limitations are likely to similarly apply to the results from both time periods in this analysis.
Conclusions: This real-world analysis suggests that no clear standard of care exists in the frontline or R/R FL setting despite the approval of novel agents. In addition, 2-year OS outcomes in the frontline and R/R settings appear to have remained similar in the periods of 2015 to 2017 and 2018 to 2021, substantiating the continued need for and increased utilization of novel therapies that might offer improvement in patient outcomes across LOTs for FL.
Disclosures
Phillips:BMS: Consultancy, Research Funding; Beigene: Consultancy; Bayer: Consultancy, Research Funding; Pharmacyclics/Janssen: Honoraria; Abbvie: Consultancy, Research Funding; Curis: Consultancy; TG Therapeutics: Consultancy; Incyte: Consultancy; Epizyme: Consultancy; Kite/Gilead: Consultancy; Xencor: Consultancy; ADCT: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Eli Lilly: Consultancy; Lymphoma & Myeloma Connect: Honoraria; Celgene: Consultancy; Genmab: Consultancy. Yu:AbbVie: Current Employment, Current equity holder in publicly-traded company. Kamalakar:AbbVie: Current Employment, Current equity holder in publicly-traded company. Davies:AbbVie: Current Employment. Mutebi:Genmab: Current Employment, Current equity holder in publicly-traded company. Bains Chawla:AbbVie: Current Employment, Current equity holder in publicly-traded company. Arnette:AbbVie: Current Employment, Current equity holder in publicly-traded company. Quadri:AbbVie: Current Employment, Current equity holder in publicly-traded company. Wang:AbbVie: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.